SafeTherapeutics: Improving Patient Outcomes by Reducing Adverse Drug Reactions

Improving Patient Outcomes by Reducing Adverse Drug Reactions
Case Study
About the Customer
SafeTherapeutics’ platform is the first product that enables healthcare professionals to promptly detect potential adverse drug reactions (ADRs) in any clinical environment. The proprietary database uses symbolic AI to process all publicly available studies organized by level of evidence. The platform is the only up-to-date product that is patient-problem-centric and provides action- able data based on real evidence.
The SafeTherapeutics team is led by highly skilled individuals with diverse expertise in clinical care, drug development, finance, and cybersecurity technology. With their extensive knowledge and expertise, the SafeTherapeutics team is confident that their intelligent platform will enhance healthcare quality and reduce costs.
The Challenge
SafeTherapeutics’ platform provides clinicians with a distinctive solution by enabling them to conduct symptom-centric searches of their proprietary database. This database has been developed by utilizing various publicly available data sources, including published data, information from the FDA, and medical journals, and enables clinicians to make informed decisions about potential ADRs.
A major challenge that SafeTherapeutics encountered during development was the platform’s poor usability and low efficiency. Additionally, multiple UI and UX challenges emerged as the amount of data processed within the platform increased exponentially.

The Solution
The SafeTherapeutics team turned to Kanda to help enhance usability and process efficiency challenges. Kanda’s team undertook the following core activities to help solve the problem:
- Conducted interviews with SafeTherapeutics stake- holders and meticulously documented platform use cases and workflows to gain a better understanding of the existing UX/UI and system functionality, the company’s business goals, and the desired state of the solution.
- Held usability interviews with critical stakeholders of the product to understand their needs and usability challenges. Outputs from these activities informed design and development work.
- Conducted iterative development and testing of wireframes, making visual design refinements, and creating clickable prototypes. Testing involved remotely moderated usability sessions on the clickable prototype, followed by a round of revisions based on the findings.

The Results
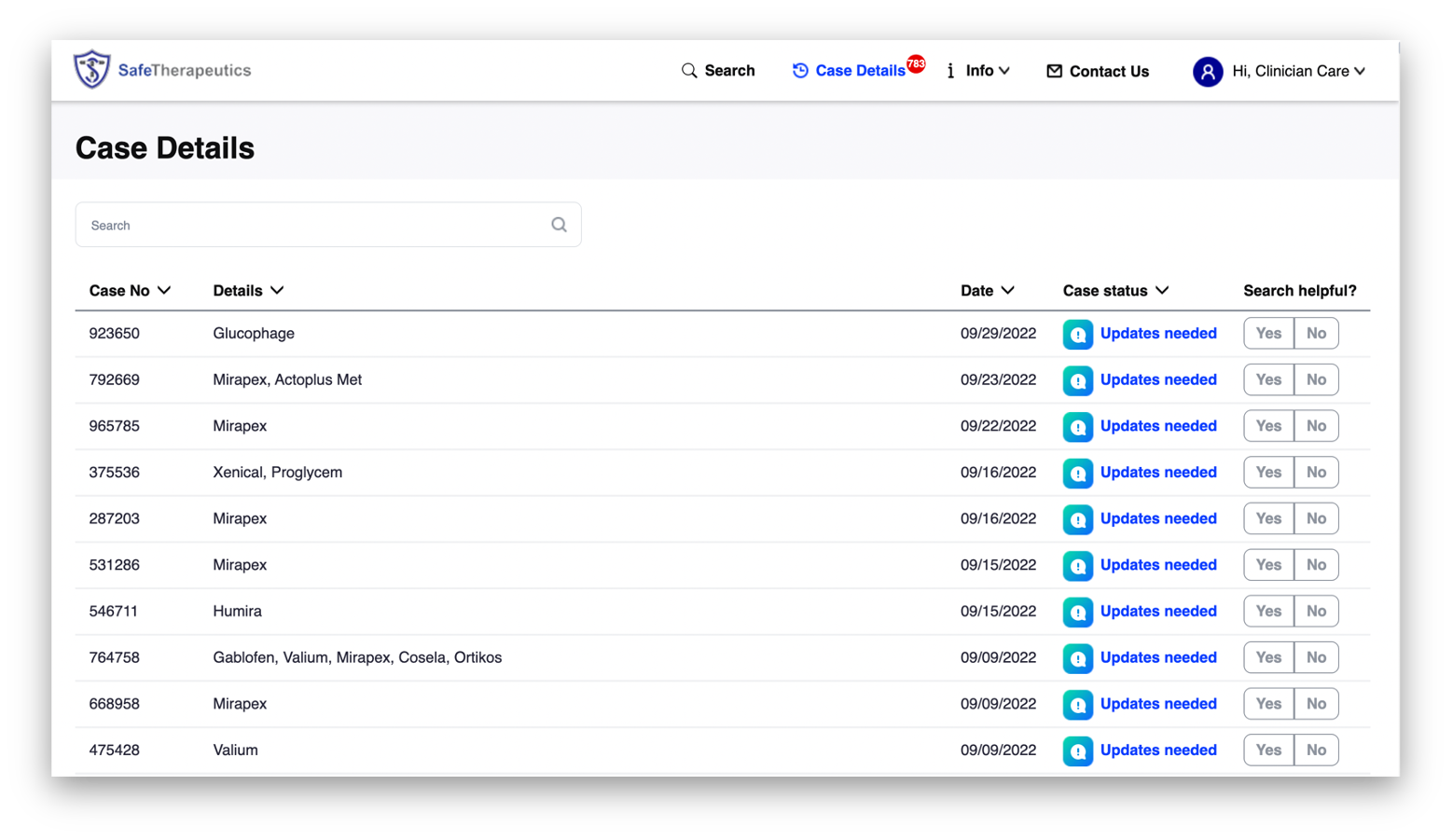
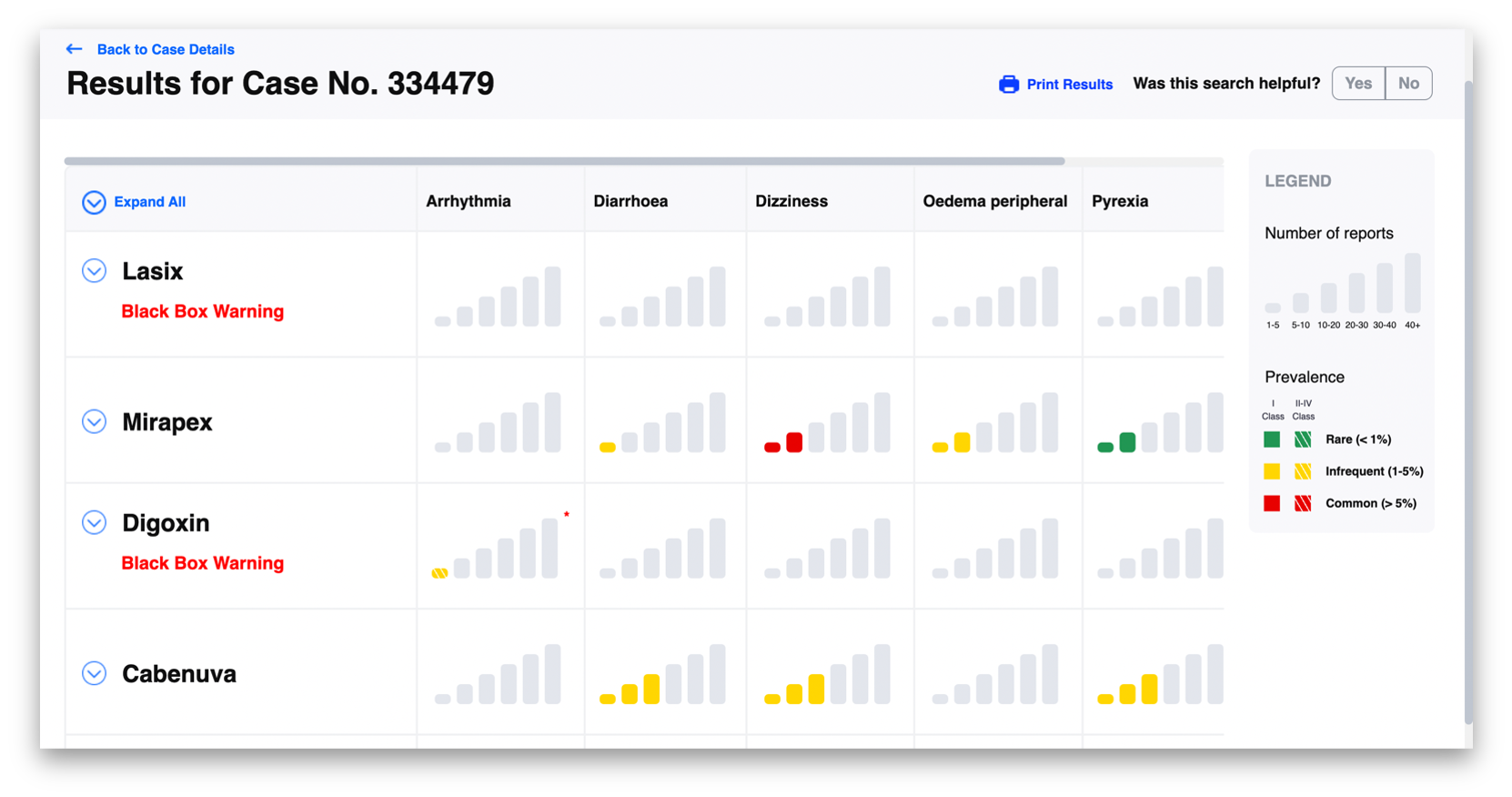
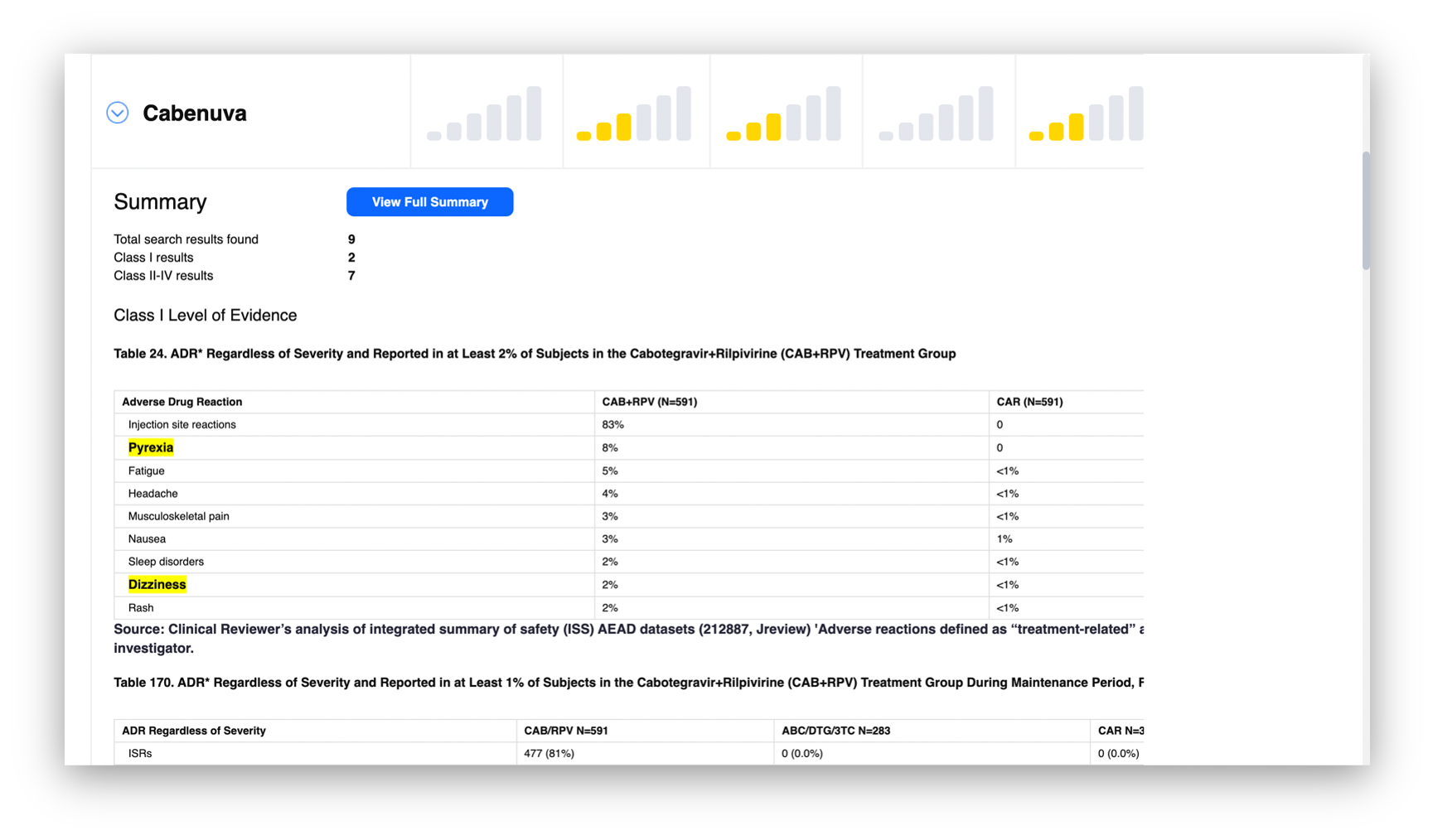
Kanda worked closely with the SafeTherapeutics team to develop front-end functionality and a new user interface for their core platform. The platform’s usability and efficiency was enhanced with a graphic display of the platform output which emphasized the frequency and highest-level of scientific evidence for any given patient problem. This enables clinician interpretation and immediate use without a learning curve: