To say that developing a new drug is a monumental achievement would be an understatement. The journey of drug discovery and development is a multifaceted, decade-long endeavor, spanning from the initial research and target molecule discovery to the final regulatory approval and manufacturing stages. This comprehensive process frequently stretches over a span of 15 years.
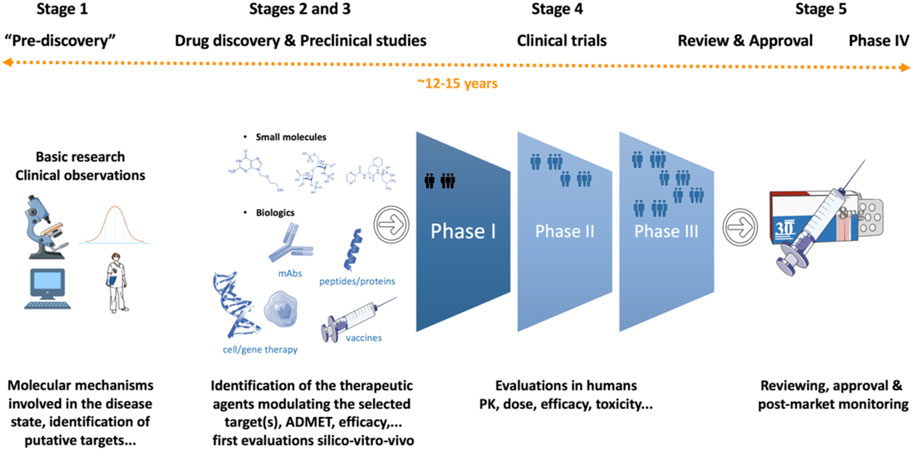
Source: Frontiers
Meanwhile, the costs tied to pharmaceutical research and development (R&D) are on a steady incline. In 2022, the pharmaceutical industry globally invested a staggering $244 billion in R&D. According to Deloitte, the average price tag for bringing a new drug to market can reach as high as $2.3 billion. That said, out of every 10,000 substances created in laboratories, only a mere 1-2 will eventually transform into real medications.
Couple this time and resource-intensive nature of the process with gradually declining returns on investment, and the pharmaceutical industry finds itself in a pressing need to discover more efficient ways for developing and introducing new drugs.
In this dynamic landscape, emerging technologies have the power to fundamentally transform the way we approach drug development and discovery. In this blog, we are going to focus on the most promising and innovative technologies that are poised to bring maximum change.
Next-generation sequencing (NGS) is a novel technology used to decipher the sequence of DNA or RNA and identify genetic variations and mutations. First introduced into commercial use in 2005, this method was initially labeled as “massively-parallel sequencing” due to its capability to concurrently sequence numerous DNA strands.
By allowing researchers to decode entire genomes faster and more cost-effectively than ever before, NGS offers numerous opportunities for the pharmaceutical industry — from accelerating the discovery of new therapeutic targets to streamlining the design of clinical trials on target populations to uncovering new indications of existing drugs.
There are already numerous examples that NGS is becoming a cornerstone of modern drug development:
Up to this point, animal studies remained the gold standard for confirming drug effectiveness in pharmaceutical development. Yet, the trustworthiness and consistency of the results from these studies are jeopardized when applied to humans, mainly due to inherent differences between animal and human systems. As a result, almost 40% of newly developed drugs do not make it through clinical trials, even after successfully passing preclinical assessments with animal models.
In addition, in December 2022 the FDA Modernization Act 2.0 marked a significant step away from mandated animal testing in favor of innovative non-animal alternatives. Organ-on-a-chip (OOC) technology has emerged as a promising solution to the challenge, providing more physiologically relevant models for drug testing and disease research compared to traditional cell cultures and animal models.
OOC technology involves the development of microfluidic devices that simulate the structure and function of human organs and tissues on a miniature scale. These devices can replicate the physiological and mechanical aspects of specific organs, allowing researchers to study responses to drugs, toxins, and diseases in a controlled laboratory setting.
Another novel approach to solving pharmaceutical R&D challenges is nanotechnology which, as the name suggests, deals with structures at the nanometer scale. Advances in nanotechnology in the medical domain even resulted in a new field called nanomedicine.
The hallmark of nanomedicine is targeted delivery. Nanoparticles and nanocarriers are engineered to encapsulate and transport drugs to specific sites within the body. This method minimizes contact with healthy tissues, curbs side effects, and maximizes the drug’s therapeutic impact. It also allows for sustained drug release, improving dosing convenience and patient compliance.
Today, there are about 100 nanotechnology-based formulations and products approved by the FDA and EMA. Pfizer-BioNTech and Moderna COVID-19 vaccines are the latest examples of nanomedicine formulations that received regulatory approval.
Three-dimensional (3D) printing stands out as another progressive technology that offers immense potential for streamlining the drug development journey. To begin with, 3D printing empowers researchers to create realistic tissue constructs, often termed organoids, replicating the intricate structure and function of human organs. These organoids prove invaluable as in vitro tools for disease modeling, enabling scientists to explore the biochemical, genetic, and histological effects of specific drugs. In the process, they yield vital insights into pharmacokinetics, pharmacodynamics, and toxicity.
In addition, 3D printing is used to produce drug delivery systems tailored to specific patient needs. This technology allows for the development of precise drug-release profiles, ensuring that medications are administered effectively and with minimal side effects. Back in 2015, the FDA granted approval to Spritam®, marking the debut of the first 3D-printed pharmaceutical. Since that milestone, the 3D-printed drug market has been steadily expanding, and it is projected to maintain an impressive CAGR of 15% until 2030.
In an attempt to maximize their R&D investments, big pharma companies turn to artificial intelligence and establish their own AI divisions. What’s more, these pharma heavyweights partner with tech giants to accelerate their R&D innovation and boost productivity. For instance, four leading pharmaceutical companies — AstraZeneca, Merck, Pfizer and Teva — joined forces with AWS to create AION Labs, an alliance that spearheads the adoption of cloud-powered AI for more efficient drug discovery and development.
Indeed, from facilitating de novo drug design to therapeutic target discovery to analyzing clinical data, and more, AI reshapes every step of the pharmaceutical product development lifecycle, dramatically accelerating the overall timeline. To wit, Canada-based Deep Genomics used its proprietary AI Workbench platform to identify a novel drug candidate for rare Wilson disease only within 18 months.
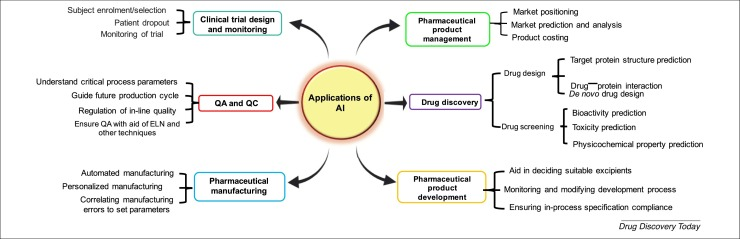
Source: National Library of Medicine
Pharmaceutical R&D is the foundation upon which we build our understanding of human health, expand our knowledge of diseases, and forge a path to safer and more effective drugs and medicines. Although the drug development process is complex and multifaceted, the latest pharmaceutical innovations are poised to simplify and accelerate this journey. From next-generation sequencing and nanotechnology to organ-on-a-chip and 3D bioprinting, these cutting-edge technologies are transforming the landscape of pharmaceutical research.
For over 25 years, Kanda has been serving well-established healthcare institutions and healthtech startups alike, delivering exceptional healthcare solutions that streamline clinical and pharmaceutical workflows, optimize patient care, and drive better outcomes. If you are looking for a trusted partner for your next healthcare development project, drop us a line, and our experts will get back to you to discuss how to turn your idea into reality.